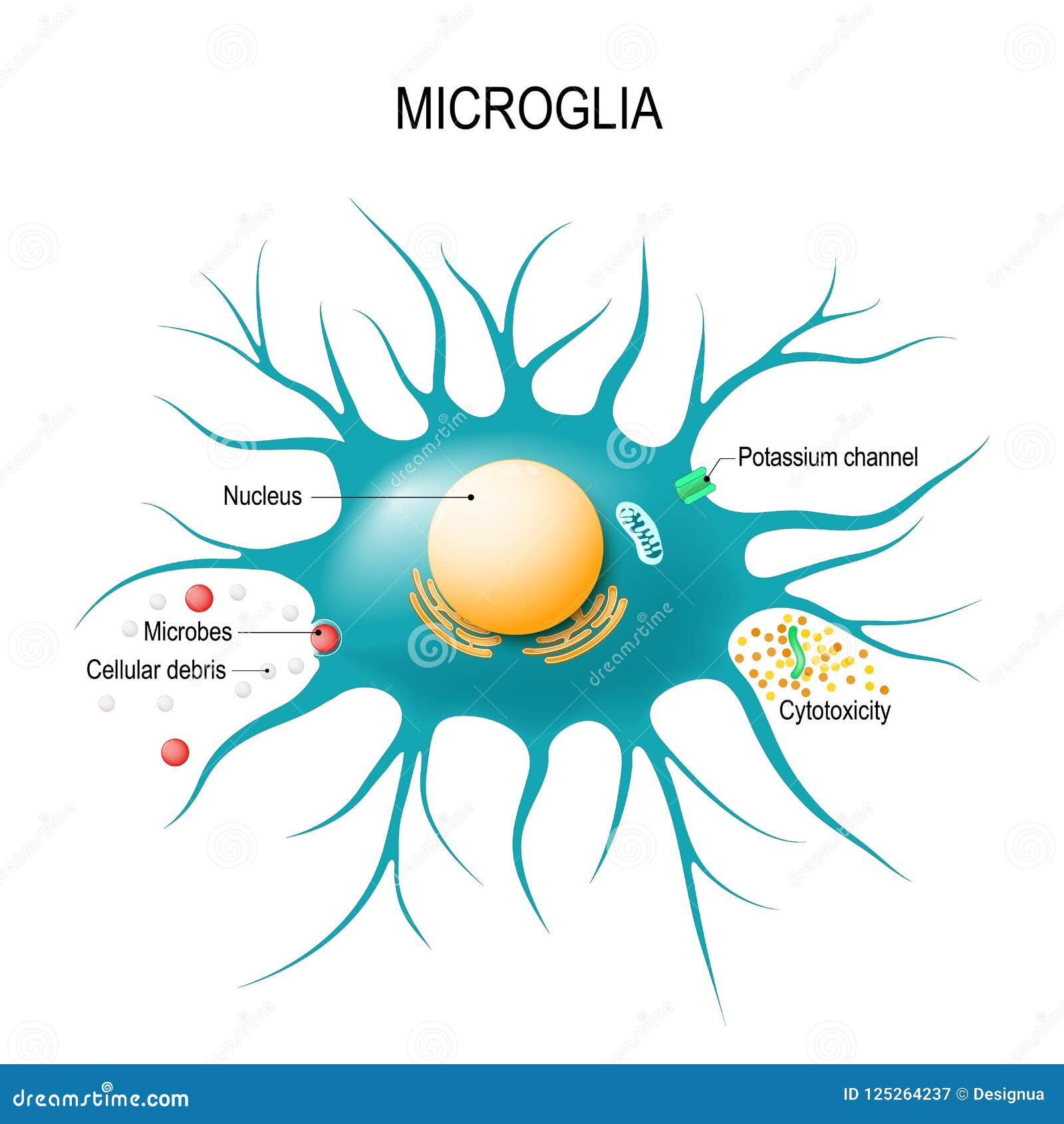
Microglial cells are the brain’s primary immune defenders, playing a critical role in maintaining brain health and responding to neurological conditions. These specialized cells continually survey the brain for potential threats, such as damaged neurons or the build-up of toxic proteins associated with neurodegenerative diseases like Alzheimer’s. Notably, neuroscientist Beth Stevens has pioneered research into how microglial cells contribute to both the protection and potential degeneration of the brain, uncovering pathways that may lead to innovative treatments. By uncovering the complexities of brain immunity, her work has opened doors to groundbreaking neuroscience breakthroughs that could benefit millions affected by cognitive disorders. As we delve deeper into the mechanisms of microglial function, we inch closer to new biomarkers and therapies that may one day revolutionize Alzheimer’s research.
The intricate network of glial cells in the central nervous system is vital for maintaining optimal brain function, and amongst them, microglia stand out due to their crucial protective roles. These brain-resident immune cells not only defend against infections but also participate in sculpting neuronal connections, highlighting their dual purpose in both health and disease. Leading researchers, such as Beth Stevens, have been instrumental in exploring the dynamics of these versatile cells, shedding light on their involvement in various neurological disorders, including Alzheimer’s and Huntington’s disease. This exploration into the realm of brain immunity represents a key area in contemporary neuroscience and holds significant promise for discovering new therapeutic strategies. By studying how these immune cells function and sometimes malfunction, scientists are carving a path toward understanding neurodegenerative diseases and improving the lives of those affected.
Understanding Microglial Cells in Alzheimer’s Research
Microglial cells are a type of glial cell located in the brain and spinal cord, playing a crucial role in the brain’s immune defenses. Research led by neuroscientist Beth Stevens has uncovered their significant involvement in neurodegenerative diseases, particularly Alzheimer’s. These cells act as sentinels that patrol neural pathways, ready to respond to signs of injury or illness. However, when their pruning processes malfunction, they can inadvertently contribute to the pathogenesis of Alzheimer’s, leading to cognitive decline and neural degeneration.
The groundbreaking work on microglial cells has opened new avenues for Alzheimer’s research. By understanding how these immune cells interact with synapses, researchers are developing potential biomarkers for early detection of Alzheimer’s and other neurodegenerative diseases. This research redefines our approach to brain immunity, emphasizing the delicate balance between support and dysfunction within the brain’s cellular landscape.
The Role of Microglia in Neurodegenerative Disease
Neurodegenerative diseases such as Alzheimer’s are complex and multifactorial, posing challenges to both researchers and clinicians. The role of microglial cells has emerged as a vital part of understanding these diseases. In healthy conditions, microglia assist in maintaining synaptic health, but in the context of neurodegenerative disorders, they can exhibit neurotoxic behaviors. According to Stevens, aberrant pruning carried out by these cells can exacerbate synaptic loss and lead to neuroinflammation, which further damages neuron function.
The insights gathered from studying microglia, as articulated by Stevens, provide a promising foundation for future therapies. By targeting the pathways that regulate microglial activation, novel treatments could be developed to modulate their activity, potentially slowing or halting the progression of diseases like Alzheimer’s. This underscores the importance of continued research and the translation of laboratory findings into clinical practice, illustrating a glimmer of hope for millions affected by these debilitating conditions.
Innovative Approaches in Neuroscience Breakthroughs
Recent advancements in neuroscience have revolutionized our understanding of brain function and its relationship to diseases such as Alzheimer’s. Stevens emphasizes that curiosity-driven research is essential for these breakthroughs. The contributions of federal funding, particularly from the National Institutes of Health (NIH), have been pivotal in supporting fundamental studies that explore the intricacies of brain immunity and the role of microglia in synaptic formation and pruning.
These innovative approaches have far-reaching implications, enabling scientists to uncover mechanisms of diseases that were previously beyond reach. Stevens’ work exemplifies how investigating the brain’s immune system can lead to groundbreaking discoveries that inform treatment strategies for Alzheimer’s and other neurodegenerative conditions. As neuroscience continues to unravel the complexities of the brain, the potential for therapeutic interventions grows stronger, bringing renewed hope to patients and families affected by Alzheimer’s.
The Impact of Basic Science on Alzheimer’s Treatment
The transformation in understanding Alzheimer’s disease has been significantly driven by basic science research. Basic science, as seen in Stevens’ journey, has laid the groundwork for much of the advancements in neurobiology today. This fundamental knowledge translates complex concepts into tangible applications that can lead to innovations in Alzheimer’s treatment. Through prospective studies, Stevens has provided critical insights into how microglia function in the healthy and diseased brain, offering pathways toward prospective therapies.
The progression from basic to applied science reflects the essence of research—demanding patience and persistence as discoveries unfold. With an understanding of microglial dysfunction, the potential for developing therapies that fine-tune the immune system’s response paves the way for novel treatments for Alzheimer’s. This illustrates a broader trend in the field, confirming that foundational discoveries can yield significant implications for managing neurodegenerative diseases.
Beth Stevens: A Pioneer in Neuroimmunology
Beth Stevens stands out as a pioneering force in the field of neuroimmunology, forging new paths in Alzheimer’s research. Her innovative ideas and rigorous experimental approaches have not only increased our understanding of microglial cell function but have also challenged the long-standing perceptions of these cells’ roles in brain health. Being named a MacArthur genius is a testament to her substantial contributions, and her perspective on curiosity-driven science informs her research ethos.
Through her leadership at the Stevens Lab, she has cultivated an environment where fundamental curiosity fuels scientific inquiry. Collaborations across institutions like Boston Children’s Hospital and the Broad Institute emphasize the importance of multidisciplinary research in addressing the complexities of neurodegenerative diseases. Stevens’ work exemplifies how a strong foundation in basic science can illuminate the pathways to understanding and eventually treating debilitating conditions like Alzheimer’s.
Challenges in Translating Research into Treatments
One of the significant challenges faced by scientists and clinicians is the translation of basic research into effective treatments for Alzheimer’s disease. Despite the remarkable advances in understanding microglia’s role in neuroimmunity, bringing these findings to clinical practice requires extensive validation and testing. Stevens points out that although the path from discovery to implementation is often lengthy, the potential implications for patient care are profound.
The translation of neuroscience breakthroughs into therapies must navigate various hurdles, including regulatory approval, funding for clinical trials, and the complexities of human biology. However, with continued support and investment in research, like those from NIH, the scientific community can aspire to overcome these obstacles. The ultimate goal remains clear: to convert discoveries into actionable treatments that can significantly improve the lives of those affected by Alzheimer’s and other neurodegenerative diseases.
The Future of Alzheimer’s Research
As we look to the future, Alzheimer’s research is poised for substantial growth and innovation, driven by advances in technology and our understanding of the brain’s immune system. The role of microglial cells will likely remain central, as researchers push the envelope in identifying biomarkers that can signal the disease long before symptoms appear. This early detection could revolutionize the way neurodegenerative diseases are approached and managed.
Innovative technologies such as CRISPR gene editing and advanced imaging techniques hold promise for further uncovering the mechanisms of Alzheimer’s. The continued collaboration across research institutions will also enhance the understanding of how microglia interact with neurons and other glial cells, providing a deeper insight into potential therapeutic targets. The resilience and ingenuity of the scientific community herald a new era in Alzheimer’s research, prioritizing effective solutions that alleviate the burden of this neurodegenerative condition.
The Importance of Funding in Neuroscience
Funding plays a vital role in the advancement of neuroscience research, influencing the depth and breadth of studies conducted on diseases like Alzheimer’s. Federal agencies, particularly the NIH, provide crucial support that enables scientists like Beth Stevens to pursue their groundbreaking investigations into microglial function and their implications for neurodegenerative diseases. Stable funding streams allow for sustained research efforts and the ability to explore bold new ideas.
Moreover, funding fosters collaboration across multidisciplinary teams which is essential for tackling complex questions in neuroscience. As the aging population increases, the pressing need for innovations in Alzheimer’s care becomes even more critical. Continued investment in foundational research will empower scientists to make transformative discoveries, ultimately leading to effective treatments and interventions for millions affected by Alzheimer’s.
Advancing Knowledge on Neuroinflammation and Alzheimer’s
Neuroinflammation is increasingly recognized as a key contributor to the onset and progression of Alzheimer’s disease. Understanding the intricate relationship between inflammation and neurodegeneration has grown significantly thanks to research on microglial cells. These cells not only respond to injury but can also initiate inflammatory processes that might accelerate synaptic damage and cognitive decline. Stevens’ work offers crucial insights into how modulating microglial activity could influence the neuroinflammatory landscape in Alzheimer’s.
As research evolves, pinpointing the mechanisms behind microglial-induced neuroinflammation will be essential for developing targeted therapies. The promise of neuroprotective treatments hinges on a deeper understanding of how these cells function within the brain’s environment. Advancements in this field can lead to groundbreaking discoveries, ultimately contributing to better management strategies for Alzheimer’s and enhancing quality of life for those impacted by this devastating disease.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are crucial in Alzheimer’s research as they function as the brain’s immune system, patrolling for signs of illness and injury. They help clear out damaged cells and prune synapses, which can influence the progression of Alzheimer’s disease and other neurodegenerative diseases.
How do microglial cells contribute to neurodegenerative disease?
Microglial cells can contribute to neurodegenerative diseases like Alzheimer’s and Huntington’s when their pruning processes go awry, leading to increased synaptic loss and inflammation. This aberrant activity has been a focus of groundbreaking research by scientists like Beth Stevens.
Why are microglial cells significant for brain immunity?
Microglial cells are essential components of brain immunity, as they monitor the neural environment and respond to injury or infection. Their ability to clear debris and regulate synaptic connections is vital for maintaining healthy brain function and preventing neurodegenerative diseases.
What insights have Beth Stevens’ research on microglial cells provided into Alzheimer’s disease?
Beth Stevens’ research has revealed significant insights into how microglial cells alter synaptic pruning, which can lead to Alzheimer’s disease. This groundbreaking work has paved the way for developing new biomarkers and potential treatments for various neurodegenerative disorders.
How have neuroscience breakthroughs influenced the understanding of microglial cells?
Neuroscience breakthroughs have significantly advanced our understanding of microglial cells, highlighting their roles beyond traditional immunity. Research has shown that microglia not only protect the brain but also actively shape neural circuits, impacting the development and progression of neurodegenerative diseases like Alzheimer’s.
Can microglial cells be targeted for new Alzheimer’s treatments?
Yes, targeting microglial cells represents a promising strategy for developing new Alzheimer’s treatments. By understanding their role in synaptic pruning and inflammation, researchers aim to create therapies that can modify microglial behavior to protect against neurodegenerative diseases.
What are the implications of microglial dysfunction in Alzheimer’s disease?
Microglial dysfunction can exacerbate neurodegenerative diseases like Alzheimer’s by failing to clear toxic proteins and promoting inflammatory responses. Understanding these mechanisms is critical for developing effective therapies to slow down or halt disease progression.
What are the key findings from Beth Stevens’ lab regarding microglial cells?
Key findings from Beth Stevens’ lab highlight that abnormal microglial activity, particularly in synaptic pruning, can lead to the development of Alzheimer’s disease. These insights are crucial for guiding future research and therapeutic strategies in neurodegenerative disease.
| Key Point | Description |
|---|---|
| Role of Microglial Cells | Microglial cells act as the brain’s immune system, patrolling for signs of illness and injury, removing dead cells and pruning synapses. |
| Impact on Alzheimer’s Disease | Aberrant pruning by microglial cells has been linked to Alzheimer’s and other neurodegenerative disorders. |
| Foundational Research | The research led by Beth Stevens has laid groundwork for new biomarkers and treatments for neurodegenerative diseases. |
| Significance of Federal Funding | Success in microglial research relies heavily on federal funding, notably from the NIH. |
| Curiosity-Driven Science | Basic science enables discoveries that lead to significant advancements in understanding and treating diseases. |
Summary
Microglial cells play a crucial role in brain health by acting as the primary immune defenders of the central nervous system. This research illustrates their importance not only in maintaining normal brain function but also in the pathogenesis of diseases like Alzheimer’s. Through ongoing studies led by experts like Beth Stevens, we can continue to uncover the complex relationships microglial cells have with diseases, leading to groundbreaking discoveries that may improve the lives of millions suffering from neurodegenerative conditions.