Funding cuts patient safety in the realm of medical research, posing significant risks to individuals participating in clinical trials. Recent disruptions, particularly the $2 billion freeze of federal research funds, highlight the crucial relationship between financial support and patient welfare. Institutions like Harvard, which depend heavily on NIH funding, face grave challenges in maintaining the necessary oversight and ethical protocols essential for safeguarding participant rights. Without robust funding, the integrity of Institutional Review Boards (IRBs) and their ability to monitor research ethics and participant safety falters, potentially paving the way for increased risks in studies. As we navigate the turbulent landscape of medical research funding, the impact on patient safety must remain at the forefront of our discussions, ensuring that the protection of research participants is prioritized.
The recent tightening of funds allocated to medical research casts a long shadow over the safety of trial participants. When financial support is restricted, the implications for patient protection become dire, particularly as oversight mechanisms like Institutional Review Boards (IRBs) struggle to fulfill their critical roles. This scarcity not only undermines the ethical foundations of research but also raises concerns about the overall integrity of clinical studies. Additionally, the relationship between funding levels and medical research efficacy sheds light on the growing challenge of maintaining participant safety amidst an evolving landscape of regulations and ethical standards. As we explore alternative phrases, it’s clear that the financial health of medical research directly affects how we safeguard the rights and well-being of individuals engaged in vital studies.
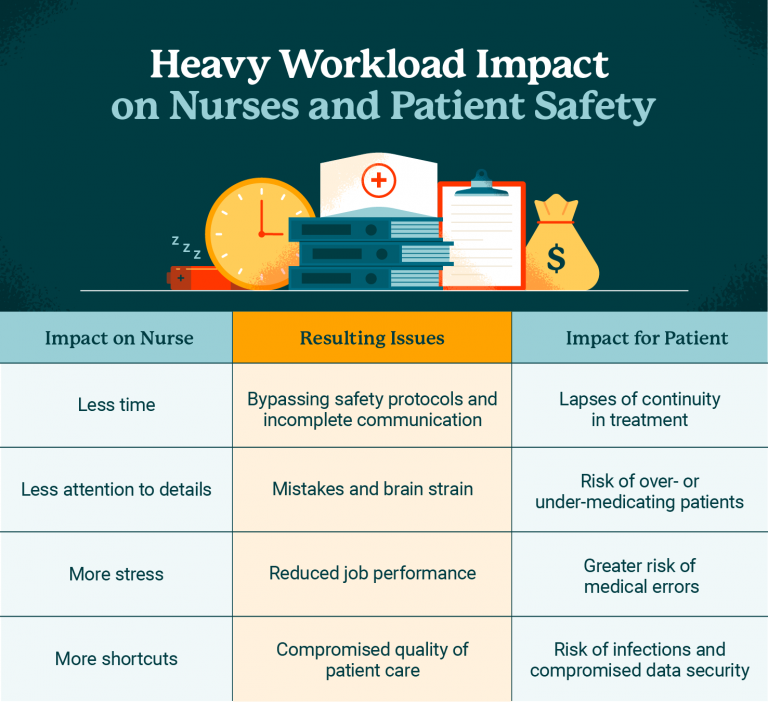
The Consequences of Funding Cuts on Patient Safety
Funding cuts in medical research present a critical challenge to patient safety, particularly in clinical trials. As federal grants diminish, institutions like Harvard face substantial obstacles in maintaining rigorous oversight and ethical standards necessary for protecting research participants. The STOP-Work order from the Trump administration that halted over $2 billion in federal research grants exemplifies how funding interruptions can disrupt ongoing studies. Institutions rely on these essential funds to support the infrastructure of IRBs (Institutional Review Boards) that ensure participant rights and safety are prioritized. Without sufficient funding, these boards are unable to adequately evaluate research proposals, leading to potential risks that could endanger participants in trials.
Additionally, ongoing funding cuts result in delayed studies and hinder the ability to recruit new clinical sites, further compromising patient safety. Research institutions struggle to navigate the increased scrutiny and administrative burdens while maintaining effective oversight. The ripple effect of these cuts can ultimately lead to a decline in public trust towards medical research initiatives. When ongoing research is interrupted, the integrity of data can be at stake, and emerging treatment options could face significant delays, ultimately prolonging patient suffering and undermining the potential benefits of clinical advancements.
The Role of NIH Funding in Enhancing Patient Protection
NIH funding plays a vital role in reinforcing the infrastructure of medical research, ensuring robust safeguards for patient protection. The federal funding mandates that research involving human participants undergo rigorous IRB review, holding institutions accountable to uphold ethical standards. This oversight system is crucial in maintaining the trust of patients who voluntarily participate in studies, making them confident their rights and well-being are protected. With substantial NIH funding, research institutions can support multidisciplinary studies more effectively, further strengthening patient protections.
Moreover, NIH policies have transitioned towards using single IRBs (sIRB) for multisite studies, which has streamlined the process of review and oversight. This shift not only enhances efficiency but also emphasizes the central role of ethical oversight in safeguarding patients’ interests. With adequate funding, institutions can train their IRB members and researchers, ensuring they are equipped to manage human subject protections effectively. The impact of NIH funding is thus far-reaching, enhancing the overarching commitment to patient safety in medical research landscapes across the nation.
How IRB Oversight Safeguards Participants in Research
Institutional Review Boards (IRBs) are fundamental to the ethical conduct of medical research, acting as guardians of participant safety. Their responsibilities include evaluating the study’s design, ensuring informed consent is obtained, identifying potential risks, and monitoring adverse events. This institutional framework is pivotal, given the historical measures that have led to the establishment of stringent regulations in human research. The balance IRBs create is essential in fostering trust among research participants, allowing for ethical exploration of new therapies while maintaining the highest standards of safety.
The IRB process is not just a tick box for compliance; it embodies a culture of ethics within medical research organizations. They work to educate researchers about ethical practices and evolve with community needs and emerging data. This critical oversight helps mitigate harm and ensure that participant welfare is prioritized, allowing research to be conducted responsibly. In a funding-constrained environment, the role of IRBs becomes even more crucial in maintaining the ethical conduct necessary for advancing medical science without compromising participant safety.
The Importance of Research Ethics in Clinical Trials
Research ethics serve as the backbone of clinical trials, emphasizing the need for integrity, respect, and care towards participants. Every phase of research involves ethical considerations, from conceptualization to publication, ensuring that participants are not only informed but also protected from harm. The historical context of unethical research practices, including infamous cases like the Tuskegee Syphilis Study, highlights the dire need for robust ethical oversight. This ethos is embodied within IRBs that scrutinize and approve research protocols, ensuring the adherence to ethical standards in conducting trials.
Moreover, ethical principles extend beyond compliance; they enrich the research experience and promote participant engagement. When patients feel assured of their safety through transparent communication and ethical practices, they are more likely to participate willingly in trials. This participation is crucial for the advancement of medicine. However, funding cuts pose a significant risk to maintaining these ethical standards. Reduced resources could compromise the ability of researchers to adhere to ethical guidelines, thereby jeopardizing not just participant welfare but also the integrity of scientific research.
Diminished Funding and Its Impact on Trust in Medical Research
Funding cuts can have far-reaching consequences on public trust in medical research. When federal funding is reduced, research institutions struggle to uphold their commitments to transparency and participant safety. This disruption can foster skepticism among potential participants, who may question the integrity and motives behind clinical trials. Public trust is essential for recruitment and for the successful execution of clinical research; if individuals do not believe that their safety is prioritized, they may opt out of participation, hindering the advancement of medical knowledge.
Trust, once lost, can take years to repair. Research programs that have been interrupted due to funding cuts may find difficulty in re-establishing community relationships. As patient involvement decreases, the generalizability of research findings may be compromised, skewing results and reducing the overall efficacy of new treatments that could benefit the population. Thus, it is vital to protect the integrity of funding for medical research to maintain public trust and ensure a committed partnership between researchers and the communities they serve.
The Consequences of Delayed Research Studies on Patient Care
When research studies are delayed due to funding cuts, the repercussions extend far beyond the academic realm; they directly impact patient care and treatment breakthroughs. Studies aimed at finding new therapies for chronic conditions or life-threatening diseases often rely on timely completion of research protocols. Delays can result in longer waiting periods for new treatments or drugs, ultimately affecting patient outcomes. For individuals relying on clinical trials for access to potentially life-saving interventions, funding interruptions represent a serious obstacle.
Moreover, prolonged research timelines can demoralize both researchers and participants involved in these studies. Participants become discouraged if they perceive a lack of movement or progress and may feel their contribution is undervalued. Researchers are faced with the dilemma of navigating a reduced resource environment, which can stifle innovation and creativity in finding solutions to pressing healthcare issues. Therefore, it is crucial to secure sustained funding for research initiatives to foster a responsive and effective healthcare system.
How Funding Cuts Lead to Inequities in Health Research
Funding cuts greatly exacerbate existing disparities in health research, thereby limiting access to clinical trials for marginalized communities. With less funding available for outreach and recruitment, underrepresented populations may miss out on opportunities to participate in research that could directly benefit their health. This inequity not only affects the diversity of research samples but also compromises the applicability of research findings to broad segments of the population. As a result, communities with the highest health disparities remain excluded from the advancements that research could bring.
The implications of these inequities are significant. If clinical trials lack diverse representation, the resulting therapies may not effectively address the unique health needs of different populations. This lack of inclusivity reinforces systemic health inequities, making it paramount that funding mechanisms prioritize diversity in research participation to ensure that advancements in medical science benefit all individuals uniformly. Advocating for dedicated funding to enhance representation and inclusivity in research is essential in addressing these disparities.
The Future of Medical Research Amidst Budget Cuts
The future of medical research hangs in a delicate balance, influenced heavily by current budget cuts. As funding trails off, research institutions face increasing pressure to justify their expenditures while simultaneously striving to maintain the quality and integrity of their studies. Innovations in treatment and care delivery rely heavily on continuous funding streams that facilitate collaboration among researchers, institutions, and communities. If this funding landscape remains precarious, the potential for groundbreaking discoveries that could alleviate suffering diminishes.
Moreover, the sustainability of research initiatives is threatened without a concerted commitment to restoring financial support for medical research. Policymakers must recognize the critical role that funding plays in not only enabling research but also in protecting patient safety through rigorous ethical oversight and collaborative partnerships. The restoration and enhancement of funding for medical research are not merely fiscal decisions; they are imperative to uphold the social contract with patients who participate in studies, trusting that their safety, welfare, and rights will remain protected in the pursuit of medical advancement.
Frequently Asked Questions
How do funding cuts impact patient safety in medical research?
Funding cuts severely disrupt the oversight mechanisms vital for patient safety in medical research, such as Institutional Review Boards (IRBs). Reduced financial resources can lead to fewer staff members, diminishing the comprehensive reviews needed to ensure participant protection. This ultimately compromises the ability to mitigate risks, assess study designs effectively, and provide necessary monitoring, thus risking patients’ health and safety.
What role does NIH funding play in ensuring patient safety?
NIH funding is crucial in sustaining research projects that prioritize patient safety. It supports the infrastructure for rigorous ethical reviews by IRBs, which are responsible for approving and monitoring clinical studies. Without adequate NIH funding, the necessary oversight and ethical standards to protect research participants may be compromised, leading to potential harms.
Why are IRBs important for patient safety in research?
Institutional Review Boards (IRBs) are essential for safeguarding patient safety in research. They evaluate all aspects of clinical studies, ensuring that participant welfare is prioritized. IRBs monitor informed consent, assess the risk-benefit ratio, and oversee ongoing research to prevent adverse effects. When funding cuts occur, the effectiveness of IRBs may diminish, jeopardizing patient safety.
How do funding cuts affect the oversight of multi-site research?
Funding cuts hinder the functionality of multi-site research oversight systems like the SMART IRB. These systems streamline the review process across multiple institutions, ensuring cohesive patient safety standards. Cuts can delay studies, block new participation, and create inconsistencies in oversight, ultimately compromising the integrity of patient protections in research.
What historical events underscore the need for funding in patient safety oversight?
Historical events, such as the Tuskegee Syphilis Study and unethical medical experiments, underscore the critical need for robust funding in patient safety oversight. Financially supporting comprehensive IRB programs and ethical review systems helps uphold the standards necessary to prevent such abuses from occurring again. Funding cuts can threaten the hard-earned protections that have been established to safeguard participants in research.
What are the consequences of cuts to medical research funding on patient trust?
Cuts to medical research funding can significantly erode public trust in clinical trials and research institutions. When studies are suddenly halted or delayed due to financial constraints, it breeds skepticism among participants about the commitment to their safety. This loss of trust can dissuade individuals from participating in future research, leading to a cycle that hampers medical advancements.
How do funding cuts impact the training and support of researchers regarding patient safety?
Funding cuts reduce resources available for training researchers in ethical practices and patient safety protocols. Proper training empowers researchers to conduct studies that prioritize participant welfare and comply with regulatory standards. A deficit in funding can lead to a lack of continuous education, increasing the risk of ethical breaches and diminishing patient safety.
What can be done to mitigate the effects of funding cuts on patient safety?
To mitigate the effects of funding cuts on patient safety, stakeholders can advocate for increased government and private funding for clinical research programs, ensure ongoing training of researchers through available grants, and reinforce the importance of ethical oversight. Collaborating with communities to build transparency and trust can also help to maintain participant willingness to engage in research despite funding challenges.
| Key Point | Details |
|---|---|
| Federal Funding Cuts | Over $2 billion in federal research grants were frozen, disrupting medical study oversight. |
| Impact on Patient Safety | Funding cuts threaten the rights and welfare of patients involved in medical research. |
| Role of IRBs | Institutional Review Boards (IRBs) ensure the safety and ethical treatment of research participants. |
| Consequences of Interruptions | Delayed studies can increase risks to participants and worsen public trust in research. |
| Historical Context | Past unethical studies highlight the need for stringent oversight to protect participants. |
| Current Challenge | SMART IRB has faced operational halts, impacting the collaboration and initiation of new studies. |
Summary
Funding cuts patient safety by jeopardizing critical oversight mechanisms in medical research. The recent federal funding freeze places patients at risk, as it disrupts Institutional Review Boards (IRBs) that ensure the ethical conduct and safety of clinical trials. With vital research projects delayed and community trust eroding, the implications extend beyond academic institutions, affecting public health and confidence in future scientific advancements.