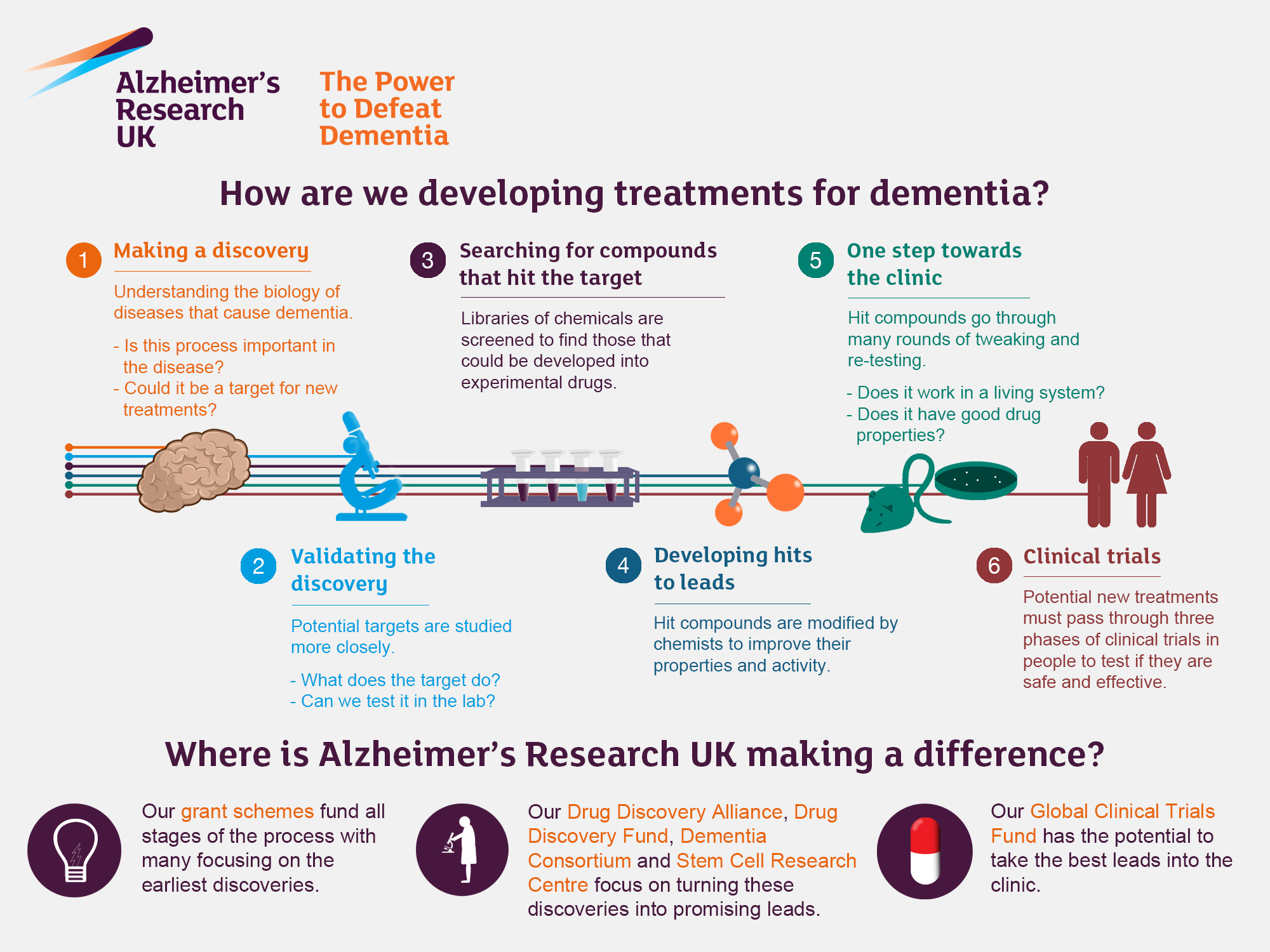
Alzheimer’s research is paving the way for groundbreaking discoveries in the fight against one of the most challenging neurodegenerative diseases affecting millions globally. Led by neuroscientist Beth Stevens, recent studies highlight the vital role of microglial cells as part of the brain’s immune system, ensuring proper functioning by removing damaged cells and pruning synapses. However, abnormal activity of these cells can contribute to the progression of Alzheimer’s, providing crucial insights for developing effective Alzheimer’s treatment options. With the number of cases expected to rise dramatically, understanding the complexities of brain health and immune responses is imperative. By focusing on innovative research, scientists aim to uncover new biomarkers and therapies that will improve outcomes for those affected by this devastating illness.
Investigating cognitive decline, especially forms such as Alzheimer’s disease, has transformed how researchers understand brain function and the immune processes at play. This ongoing inquiry involves a critical examination of glial cells, which serve as the brain’s defense mechanism, monitoring potential threats and injuries. The work of scientists like Beth Stevens emphasizes the importance of these cells in regulating synaptic health, revealing their dual role in both protecting and potentially damaging brain structures. As researchers delve deeper into the mechanisms behind neurodegenerative illnesses, the possibility of developing effective interventions becomes more promising. Moreover, advancing our knowledge in this area could lead to significant breakthroughs in treating conditions such as Alzheimer’s, fundamentally changing how we approach brain health.
Understanding Microglial Cells and Their Role in Neurodegenerative Diseases
Microglial cells play a crucial role in the brain’s immune system, constantly surveying for potential threats like pathogens and dead cells. They are integral to maintaining homeostasis in the central nervous system (CNS) because they help eliminate toxic substances and damaged neurons that can lead to neurodegenerative diseases such as Alzheimer’s. However, when microglial function goes awry, it can result in abnormal pruning of synapses, exacerbating conditions like Alzheimer’s disease and Huntington’s disease. This aberrant pruning underscores the significant impact these immune cells have on neuronal health and disease progression.
Research led by Beth Stevens has illuminated the dual nature of microglial cells, showcasing their protective capabilities alongside their potential for harm. By promoting excessive synaptic pruning, dysfunctional microglial cells can contribute to cognitive decline associated with neurodegenerative diseases. Stevens and her team are actively investigating this phenomenon to establish a clearer relationship between microglial dysfunction and the onset of Alzheimer’s disease. This understanding is critical for developing new therapeutic strategies aimed at restoring normal microglial function to combat the progression of such debilitating conditions.
Beth Stevens and Groundbreaking Alzheimer’s Research
Beth Stevens’ pioneering research into microglial cells has positioned her at the forefront of Alzheimer’s research, significantly altering the perception of how these cells affect brain health. By demonstrating that microglial cells can contribute to neurodegenerative diseases through excessive synaptic pruning, her work lays critical groundwork for understanding the underlying mechanisms of Alzheimer’s. Stevens emphasizes that curiosity-driven research not only enriches scientific knowledge but can also lead to practical applications in disease prevention and treatment, ultimately benefiting millions of individuals facing the realities of Alzheimer’s.
Her lab’s findings have immense implications for developing innovative Alzheimer’s treatments. By identifying specific pathways involving microglial cells, researchers are hopeful about creating targeted interventions that could mitigate the harmful effects of this immune response in Alzheimer’s patients. Furthermore, the evolution of potential biomarkers discovered through Stevens’ research offers promise for earlier diagnosis, enabling timely therapeutic strategies that could significantly improve the quality of life for those at risk of Alzheimer’s, as well as contributing to the efforts needed to address the growing Alzheimer’s crisis.
The Importance of Federal Support in Alzheimer’s Research
Federal funding has played a pivotal role in advancing Alzheimer’s research, particularly in the case of Beth Stevens’ groundbreaking work on microglial cells. The National Institutes of Health (NIH) has been instrumental in providing the necessary resources to explore complex biological questions that can drive significant advancements in neurodegenerative disease understanding. The early career support Stevens received, along with continued funding for her lab, illustrates how essential federal investment is to drive scientific inquiry and innovation in the field of Alzheimer’s research.
With the rapidly aging population and the rising prevalence of Alzheimer’s disease, sustaining federal support for this critical area of research is imperative. Demand for effective treatments can only be met through robust funding that encourages scientists to explore uncharted territories of brain health and disease. Stevens advocates for increased attention to foundational research, as many breakthroughs stem from seemingly distant inquiries into basic biology, emphasizing that the impact of such studies can lead to transformative changes in the treatment landscape for Alzheimer’s and other neurodegenerative diseases.
Future Directions in Alzheimer’s Treatment and Research
Looking ahead, the future of Alzheimer’s treatment lies in a multi-faceted approach that incorporates insights from neuroimmune research. As scientists like Beth Stevens identify the roles of microglial cells in neurodegenerative disease pathology, there’s an increasing focus on developing therapies that can modulate immune responses in the brain. Understanding the delicate balance microglial cells need to maintain can lead to new strategies that prevent or reverse the damage caused by Alzheimer’s. By fostering this avenue of research, we may be on the brink of discovering groundbreaking treatments that truly address disease progression.
Moreover, ongoing research in this field is likely to integrate artificial intelligence and advanced imaging techniques to monitor microglial activity in real-time, providing invaluable data for trialing new Alzheimer’s treatments. As interdisciplinary collaborations become more common, combining insights from immunology, neurology, and emerging technologies, the potential to revolutionize the care and management of Alzheimer’s increases. Continuous exploration of microglial behavior and its implications for Alzheimer’s could ultimately lead to enhancing the lives of millions by reducing the incidence and severity of this challenging condition.
Exploring Synaptic Pruning and Its Implications in Alzheimer’s
Synaptic pruning is a natural process, but when dysregulated, it becomes a key player in the development of Alzheimer’s disease. Microglial cells are responsible for this pruning process, providing an essential function during brain development and homeostasis. However, research shows that excessive pruning of synapses can lead to cognitive deficits and memory loss associated with Alzheimer’s. Understanding the mechanisms that govern synaptic pruning offers a crucial insight into how to potentially reverse synaptic loss and improve cognitive function in affected individuals.
Beth Stevens has been a leading voice in asserting that modulating microglial activity could hold the key to effective Alzheimer’s treatments. By focusing on the pathways involved in inappropriate synaptic pruning, her lab is paving the way for therapeutic strategies that could enhance synaptic integrity and neuronal communication. This fundamental research underscores the importance of addressing the underlying biological processes contributing to Alzheimer’s, rather than solely focusing on symptoms, heralding a new era in neurodegenerative disease management.
Microglia: The Brain’s Immune Soldiers in Alzheimer’s
Microglia serve as the brain’s primary immune cells, and their health is vital for combating neurodegenerative diseases like Alzheimer’s. Acting as the brain’s first line of defense, these cells monitor the environment, responding to injuries, infections, and other disruptions. However, in Alzheimer’s, these immune soldiers can become misdirected, leading to pathological conditions that contribute to neurodegeneration. Their role in synaptic pruning and maintenance makes them a critical focus in understanding how Alzheimer’s develops and progresses.
Research by Beth Stevens has highlighted how dysfunctional microglial activity not only contributes to the progression of Alzheimer’s but may also provide avenues for therapeutic intervention. By enhancing our understanding of how these immune cells interact with neurons during disease states, scientists are better equipped to design targeted treatments that can manipulate microglial function. This interplay between microglial activity and neuronal health is a pivotal area of focus, with the potential to change the treatment landscape for Alzheimer’s in the coming years.
The Significance of Biomarkers in Alzheimer’s Detection
Biomarkers play a crucial role in the early detection and progression monitoring of Alzheimer’s disease, providing insights into the biological mechanisms underlying this complex disorder. By identifying specific markers that correlate with neurodegenerative processes, scientists can develop diagnostic tools that enable earlier intervention. Beth Stevens’ research into microglial cells has opened new avenues for identifying biomarkers, offering hope that advancements could lead to more individualized treatment strategies for Alzheimer’s patients.
The importance of discovering reliable biomarkers extends beyond diagnosis; they also assist in gauging treatment effectiveness. As new potential therapies emerge from Stevens’ work on microglial function, correlating biomarker responses with clinical outcomes will be essential. This integration of biomarker data with therapeutic strategies will be critical in shaping the future of Alzheimer’s research and treatment, pushing for a more proactive and personalized approach in managing this debilitating disease.
Translating Research Into Alzheimer’s Treatment Solutions
Translating laboratory research into clinical practice remains a significant challenge in Alzheimer’s treatment development. However, Beth Stevens’ work exemplifies how foundational research on microglial function can lead to actionable therapeutic solutions. By revealing the links between microglial activity and neurodegeneration, her studies not only inform potential treatment pathways but also inspire future research directions aiming to optimize brain health. This translation of science into practice is paramount in addressing the healthcare challenges posed by Alzheimer’s.
To effectively tackle Alzheimer’s disease, collaboration among scientists, clinicians, and policymakers is necessary to ensure that the findings from research labs reach patients in meaningful ways. Stevens emphasizes that fostering a close relationship between basic research and clinical application will enhance the speed and effectiveness of developing Alzheimer’s treatments. Such collaboration can spur innovative approaches to combatting Alzheimer’s, driving forward advancements that significantly improve patient outcomes and quality of life.
The Future of Neuroimmune Research and Alzheimer’s Disease
Neuroimmune research represents a burgeoning frontier in understanding and treating Alzheimer’s disease. As scientists delve deeper into the interplay between microglial function and neuronal health, the potential for revolutionary insights increases. Beth Stevens’ pioneering work has illuminated how immune responses in the brain impact neurodegeneration, prompting a reevaluation of traditional treatment paradigms. By investigating the dual role of microglial cells in the brain, future studies could uncover new therapeutic strategies that target these cells to prevent or even reverse Alzheimer’s pathology.
Looking forward, integrating advancements in genetics, immunology, and neurobiology will be essential. Collaborations across disciplines can accelerate discoveries that inform the development of targeted interventions for Alzheimer’s. As the understanding of neuroimmune dynamics continues to evolve, the possibility of innovative therapeutic options becomes ever more feasible, holding great promise for those affected by Alzheimer’s and the overall management of neurodegenerative diseases.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are critical to Alzheimer’s research as they function as the brain’s immune system, monitoring for signs of disease and repairing damage. In Alzheimer’s, abnormal activity of these cells can lead to improper pruning of synapses, contributing to neurodegeneration. Understanding their functions can help scientists develop targeted Alzheimer’s treatments.
How is Beth Stevens contributing to Alzheimer’s research?
Beth Stevens is a pioneering neuroscientist whose research focuses on microglial cells and their role in Alzheimer’s and other neurodegenerative diseases. Her discoveries related to synaptic pruning have significant implications for developing new Alzheimer’s treatments and biomarkers for earlier detection.
How do microglial cells relate to neurodegenerative diseases like Alzheimer’s?
Microglial cells are involved in neurodegenerative diseases such as Alzheimer’s by regulating the brain’s immune response. They help clear damaged neurons and maintain synaptic health, but abnormal functioning can worsen the progression of Alzheimer’s disease, making them a key target in ongoing research.
What innovations in Alzheimer’s treatment have emerged from the study of microglial cells?
Innovations in Alzheimer’s treatment stemming from microglial cell research include the development of potential new medications that target the immune responses of these cells. This research aims to correct abnormal synaptic pruning and improve neuroprotection, potentially altering the course of Alzheimer’s disease.
Why is understanding the brain’s immune system important for Alzheimer’s research?
Understanding the brain’s immune system is pivotal for Alzheimer’s research because it reveals the mechanisms by which microglial cells influence neural health and disease. This knowledge can lead to advanced therapeutic strategies aimed at enhancing neuroinflammation control and preventing neurodegeneration, essential for combating Alzheimer’s.
What are the implications of Beth Stevens’ research for early detection of Alzheimer’s?
Beth Stevens’ research has significant implications for early Alzheimer’s detection by identifying new biomarkers linked to microglial function. These biomarkers could enable clinicians to diagnose Alzheimer’s disease at earlier stages, which is crucial for effective intervention and treatment.
How does federal funding support Alzheimer’s research advancements?
Federal funding plays a vital role in advancing Alzheimer’s research by providing resources for foundational studies. Such financial support enables researchers like Beth Stevens to explore the complex roles of microglial cells in Alzheimer’s and develop innovative treatment approaches, driving progress in the fight against neurodegenerative diseases.
| Key Points | Details |
|---|---|
| Beth Stevens’ Research | Focuses on microglial cells, the brain’s immune system. |
| Role of Microglia | Patrols the brain, eliminates dead cells, and prunes synapses. |
| Impact on Diseases | Abnormal pruning linked to Alzheimer’s, Huntington’s, and other disorders. |
| New Developments | Research paves way for new medications and biomarkers for neurodegenerative diseases. |
| Future Projections | Projected double in annual Alzheimer’s cases in the U.S. by 2050, increasing care costs significantly. |
Summary
Alzheimer’s research is crucial as it continues to unravel the complexities of neurodegenerative diseases. The innovative work of Beth Stevens highlights the importance of understanding microglial cells in the brain’s immune response and their potential role in Alzheimer’s disease. By addressing abnormal pruning processes and developing new treatment avenues, her research lays a foundation for future breakthroughs. As the population ages and the number of Alzheimer’s cases rises, the implications of this work will become even more significant, emphasizing the necessity for continued support and funding in Alzheimer’s research.